

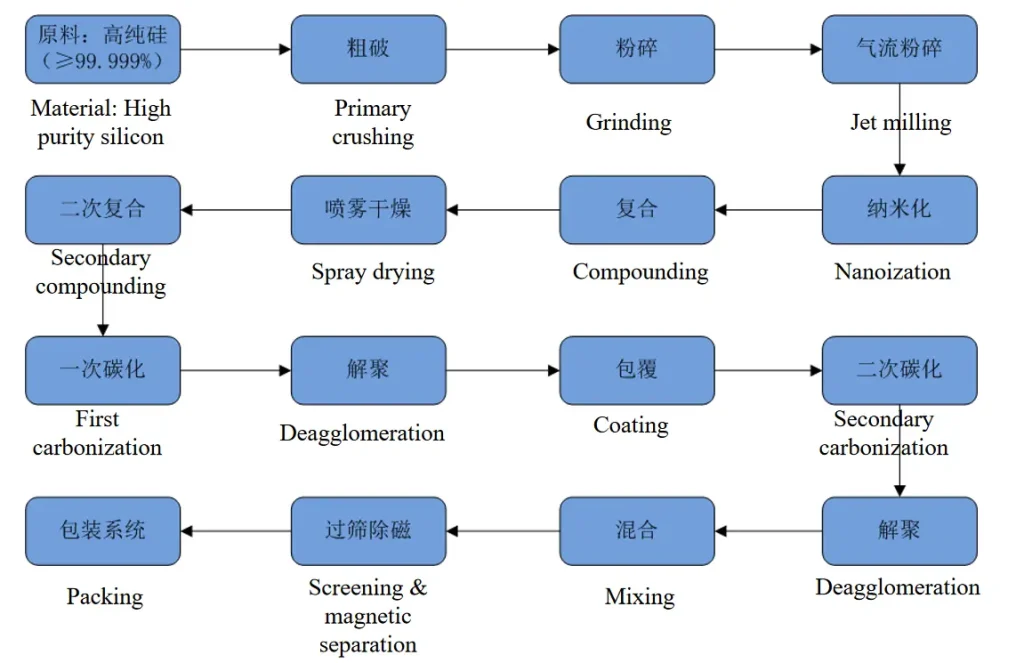
Secondary lithium ion battery is considered as the most ideal energy storage and conversion tool because of its advantages of high open circuit voltage, high energy density, long life, no pollution and small self-discharge. At present, lithium ion batteries have been widely used in portable electronic devices, electric vehicles/hybrid electric vehicles and energy storage systems, etc. With the demand of intelligent and multi-functional products, improving the energy density of lithium ion batteries has become a research focus. In lithium-ion battery system, anode and cathode materials play a decisive role in its energy density.
At present, various anode and anode materials and corresponding electrolytes have been developed and applied in lithium ion batteries. The cathode material widely used in commercial batteries is graphite, mainly including meso-phase carbon microspheres (MCMB), artificial graphite and natural graphite. Lithium ion batteries made of graphite are mainly used in portable electronic products. Modified graphite has been used in power batteries and energy storage batteries. The specific capacity of high-end graphite products on the market is close to the theoretical value of 360mA• H •g−1, and has excellent cycling performance, which is difficult to further improve. Simulation results show that increasing the specific capacity of the cathode material within 1200mA•h•g−1 is still a great contribution to improving the energy density of the battery.
At present, the main problem in the preparation of Si/ graphite composites is how to ensure the uniform and stable composite of nano-Si and graphite, so that the composites can take into account both high specific capacity and cyclic stability. In general, the preparation of Si/ graphite composites with nano-Si and graphite as raw materials needs to be combined with a variety of technical means. In this paper, we only use the one-step technique of Si and graphite combination to classify, mainly including solid-phase mixing method, liquid phase process and vapor deposition process.
一、Solid-phase mixing method
In the early stage, researchers mainly prepared Si/ graphite composites by simple mechanical mixing, namely solid phase mixing method. Although the solid-phase recombination method is simple, the combination of Si and graphite is not close, and a large amount of Si is exposed in the electrolyte, which has an adverse effect on the electrochemical performance.
For example,Cheng et al. used a high-energy mechanical ball mill to grind micron Si powder, graphite powder and multi-walled carbon nanotubes in a stainless steel ball mill tank to obtain a mixture of nano-Si/graphite/multi-walled carbon nanotubes, in which the Si content is 33wt%. Electrochemical tests showed that the first reversible specific capacity was about 2000mA•h•g−1 when the current density was 35mA•g−1, and the reversible specific capacity remained at 584mA•h•g−1 after 20 cycles.
Xu et al. prepared Si nanowire with a diameter of about 100nm by metal catalytic etching, and then directly ball-milling 15wt% Si nanowire with micron graphite powder to prepare Si nanowire/graphite anode material. The first Coulomb efficiency was 74% and the reversible specific capacity was 514mA after 15 cycles • H • G −1.Yin obtained Si/Mn/ graphite micron-grade composites by mechanical ball milling of micron-grade Si powder, Mn powder and graphite, in which the Si content was 20wt%. The first coulomb efficiency is 70%, and the reversible specific capacity is 463mA•h•g−1 after 20 cycles, when the current density is 0.15mA•cm−2.
Whittingham et al. obtained Si-Al-graphite composites by mechanical ball milling of Si powder, aluminum powder and graphite, with Si content of 7.9%. At 0.5mA•cm−2 current density, the first reversible specific capacity is 800mA•h•g−1 and the coulomb efficiency is 80%. After 10 cycles, the reversible specific capacity remains about 700mA•h•g−1.
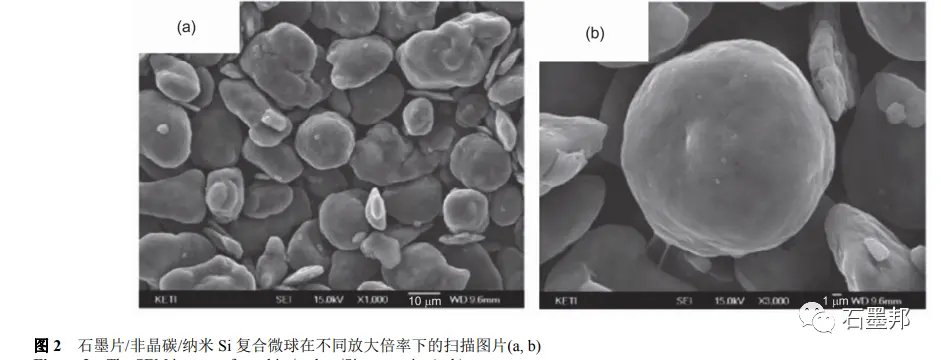
Kim et al. prepared nano-Si powder by ball milling micron Si powder and then compounded it with pitch and graphite sheet. After mechanical granulation and high temperature calcination, nano-Si/amorphous carbon/graphite spherical composite material was obtained, in which Si content was about 20%. The structure of the product is shown in Figure 2. Electrochemical tests show that the first reversible specific capacity is 560mA•h•g−1 at the current density of 140mA•g−1, the first coulomb efficiency is 86%, and the reversible specific capacity remains 80% after 30 cycles. The introduction of the third phase M(M = metal, graphene or amorphous carbon) can promote the close bonding between Si and graphite, and is conducive to increasing the electrical conductivity of the material, which provides a new design idea for the preparation of Si/ graphite composites.
二、Liquid phase complex method
The liquid phase composite process can make the raw materials disperse more evenly in a mild environment, and usually introduce the third phase substance M(amorphous carbon, graphene, metal, metal silicide, etc.) to promote the combination of Si and graphite, which is the main direction of Si/ graphite composites preparation.
Guo et al. fully dispersed nano-Si, citric acid and flake graphite in ethanol solution. After drying, they calcined at 500℃ to obtain nano-Si/amorphous carbon/graphite composites, in which amorphous carbon tightly “bonded” nano-Si to the surface of graphite, and the mass fraction of Si was about 7.2%. Electrochemical tests show that the first coulomb efficiency is about 80% and the reversible specific capacity is 476mA•h•g−1 when the current density is 0.1A•g−1, and the specific capacity remains 86% after 100 cycles.
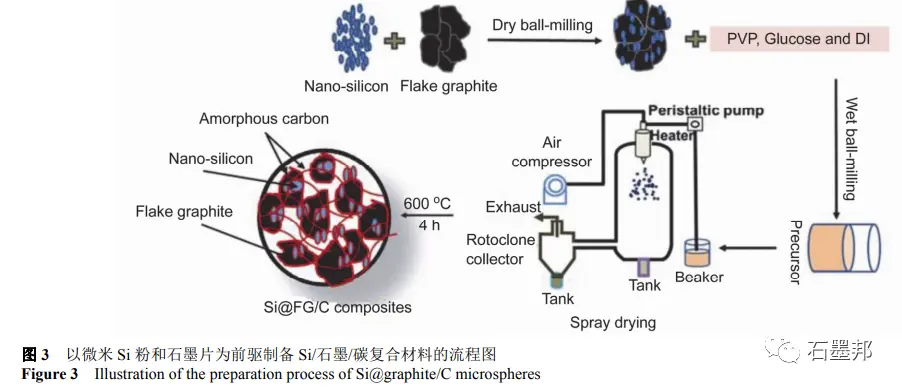
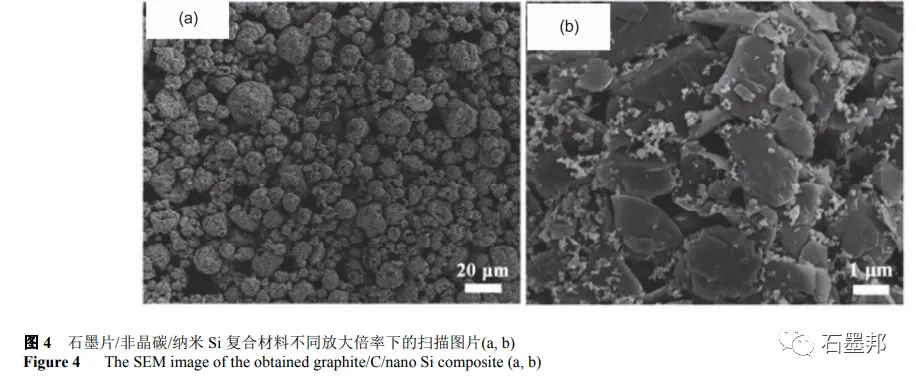
Cao et al. used commercial nano-Si powder and graphite sheet as raw materials, combined with mechanical ball milling, spray drying technology and high temperature calcination to obtain nano-Si/amorphous carbon/graphite composites, in which Si content is about 10%. Figure 3 shows a flow chart of the preparation process. The final samples obtained are micron particles composed of graphite sheets, Si nanoparticles and amorphous carbon, as shown in FIG. 4. Under the current density of 0.2A•g−1, the coulomb efficiency of the first ring is 74%, and the reversible specific capacity is 587mA•h•g−1. The reversible specific capacity is maintained at 420mA•h•g−1 for 300 cycles at A current density of 0.5A•g−1.
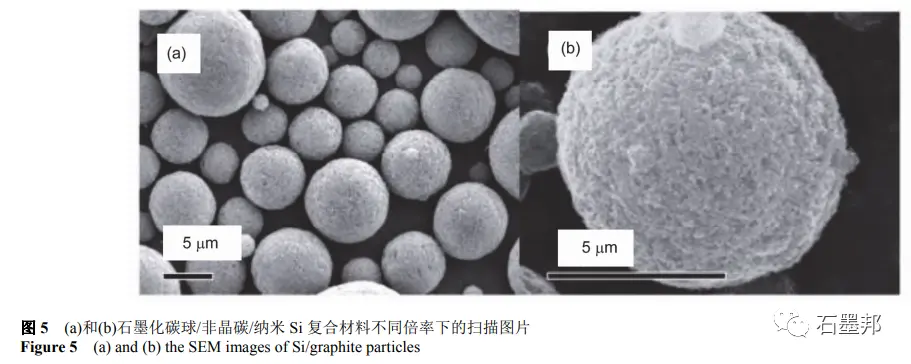
Su, such as using mechanical ball grinding micron size Si powder preparation of nanometer Si powder (100 nm), in water solution, the nano Si, glucose, graphitized carbon nano ball evenly dispersed, after spray drying granulation into micro ball precursor, after 900 ℃ calcination process in inert gas for Si/amorphous carbon/graphite composite materials, including Si content is 5 w t%. The resulting product is a micron sphere with multistage structure, as shown in Figure 5. Electrochemical measurements show that the reversible specific capacities are 435 and 380mA•h•g−1 at 500 and 1000mA•g−1, respectively. After 100 cycles of 50mA•g−1, the reversible specific capacity is 483mA•h•g−1, but the first coulomb efficiency is only 51%, mainly because nano-sized particles have large specific surfaces and form a large number of SEI films.
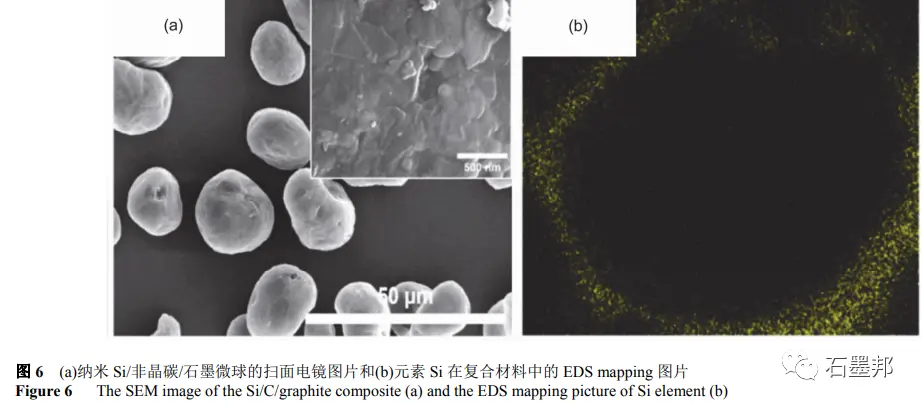
Kim et al. first dissolved coal pitch in tetrahydrofuran, and then added nano-Si powder and graphite microspheres. After ultrasonic dispersion, tetrahydrofuran is evaporated to obtain a precursor mixture, in which the ratio of Si to graphite can be controlled by adding raw materials. After calcination at 1000℃ in Ar atmosphere, amorphous carbon generated from asphalt pyrolysis “sticks” Si nanoparticles closely to the surface of graphite microspheres, as shown in FIG. 6. The final product is “potato shaped” particles, and Si nanoparticles are uniformly compound in the outer layer of graphite spheres.
When the current density is 0.15A•g−1, the first reversible specific capacity and the first coulomb efficiency of the composites with Si mass fraction of 15% are 712mA•h•g−1 and 85% respectively. After 100 cycles, the reversible specific capacity remains 80%. With the increase of Si content, the specific capacity of the composite is improved, but the cyclic stability is not so high, mainly due to the volume expansion of Si.
三、Chemical vapor deposition
Chemical vapor deposition is mainly based on graphite. Si is deposited on graphite surface by pyrolysis of silane at high temperature. The biggest advantage of vapor deposition is that Si nanoparticles can be uniformly distributed on the surface of graphite. Holzapfel et al. directly grew a layer of Si nanoparticles on the surface of graphite sheet by chemical vapor deposition (Si particle size is 10-20nm, mass fraction is 7.1%). Electrochemical tests show that the first reversible specific capacity is 520mA•h•g−1, coulomb efficiency is 75%, and the reversible specific capacity is 470mA•h•g−1 when the current density is 10mA•g−1.
Cho et al. obtained porous graphite by etching graphite microspheres catalyzed by metal nickel, and then grew Si nanowires on porous graphite by catalytic cracking silane of metal gold. Si nanowires/graphite composites were obtained with the mass fraction of Si being 20%. Figure 7 shows the simulation diagram of the preparation process. When the current density was 0.05c (1C = 1050mA•h•cm−2), the reversible specific capacity and coulomb efficiency of the first cycle were 1230mA•h•cm−2 and 91%, respectively. The reversible specific capacity was 1014mA•h•cm−2 for 100 cycles at 0.2c, and no obvious attenuation was observed.
In summary, the composite process of Si nanocrystalline graphite mainly includes solid phase method, liquid phase method and gas phase deposition method, combined with spray drying, mechanical granulation, high temperature sintering and other technical means. In general, the introduction of a third phase material (amorphous carbon, graphene, metal, metal silicide) can further promote the uniform recombination of Si and graphite, so that the two are tightly “bonded” together, while forming a three-dimensional conductive network and avoiding direct contact between the nano Si and the electrolyte.
To find out more about our products and solutions, please fll out the form below and one of our experts will get back to you shortly
3000 TPD Gold Flotation Project in Shandong Province
2500TPD Lithium Ore Flotation in Sichuan
Fax: (+86) 021-60870195
Address: No.2555,Xiupu Road, Pudong, Shanghai
Copyright © 2023. Prominer (Shanghai) Mining Technology Co.,Ltd.
