

With the intelligent and high-end social development, the proportion of new energy in the energy architecture system is increasing, especially the rapid development of new energy vehicle industry in recent years, which further promotes the reform of the energy industry. Lithium ion battery has the advantages of high energy density, good cycle performance, small self-discharge and so on. Therefore, as an energy storage device, it has been widely used in power battery, 3C digital, energy base station and other fields.
At present, the commercial anode materials are mainly amorphous carbon (soft carbon and hard carbon), graphite (natural graphite and artificial graphite), lithium titanate and silicon based materials (silicon, silicon oxide and amorphous silicon), among which the graphite anode materials for power batteries account for more than 97% of shipments. However, the 360-365 mAh·g-1 capacity of high-end graphite products is close to the 372 mAh·g-1 theoretical gram capacity of graphite. The limited space to improve the energy density of batteries is hindering further improvement, so the development of high energy density of anode materials is the key to improve the cell energy density.
Silicon-based materials with a high specific capacity of 3579 mAh·g-1 and a low electrochemical lithium embedding potential of 0.4 V(vs.Li/Li +), as well as abundant resource reserves, are considered as the most potential anode materials for the next generation of high-energy density lithium ion batteries. Relevant studies have proved that the silicon negative electrode must be used when the cell energy density is greater than 280 Wh·kg-1 without the use of lithium rich anode. However, in the process of Li+ embedding, amorphous LixSi appears on the surface of silicon particles, while the internal silicon particles remain crystalline. When the degree of lithium increases to the full formation of Li22Si5, the theoretical capacity reaches the highest 4200 mAh·g-1, and the volume expansion is 320%, much higher than the 16% volume expansion of carbon material. The bulk deformation results in the destruction and repeated formation of the solid-phase electrolyte layer (SEI), resulting in the reduction of the first Coulomb efficiency (ICE) and the loss of active lithium ions. The low proportion of silicon anode materials mixed with graphite can increase the energy density and reduce the volume effect to a certain extent, but the low ICE problem still needs to be improved by corresponding technology.
Lithium metal can be directly used as a lithium source for lithium pre-lithiation technology. Due to its low melting point (180 ℃), it is easy to be processed into lithium sheet, lithium belt, lithium particles and other forms under the condition of inert atmosphere or vacuum. At the same time, lithium metal itself is relatively soft and easy to be calendrated into film layer and attached. Therefore, the study of prelibiation using lithium metal as lithium source by different processes has attracted wide attention.
Lithium metal can be used directly in contact with the anode material or attached to the surface. Due to its low potential, lithium metal will be transformed into free Li+ in the electrolysis solution under the condition of electron exchange, and lithium embedding reaction will occur with the material. Kim et al. deposit lithium metal on the surface of the prepared silicon carbon electrode in the form of hot steam through vacuum heating deposition. At high temperature, the silicon-based material realizes lithium by directly contacting with lithium metal. At 0.1C ratio, the ICE of the full battery assembled by LiCoO2 positive electrode and pre-lithium Si-GR negative electrode increased from 76.4% to 92.5%, and the battery capacity increased from 138.2 mAh/g to 148.2 mAh/g. At the same time, the battery capacity retention rate was 80%. The number of cycles increased from 122 to 366 after pre-lithiation.
Rezqita et al. used lithium sheet as symmetric electrode and phenolic resin to prepare carbon silicon negative electrode assembly button battery under the action of external circuit, and obtained the carbon silicon material prelithium by electrochemical prelithium. The pre-lithiated silica and cathode Lini0.5Mn0.3Co0.2O2 can increase the ICE from 26% to 86%, while increasing the full battery capacity from 48 mAh/g to 160mAh/g. Yao et al. realized the pre-lithiation of silica by direct contact between lithium sheets and graphene-coated silica materials by short-circuit after the addition of electrolytic droplets. The graphene-coated silicon carbon material ICE was improved to 97.1% by short circuit prelithiation in direct contact with lithium metal for 5min. After 500 cycles of charge and discharge, the capacity of ICE was maintained at a current density of 969mAh/g at 2A/g, with good cycle stability.
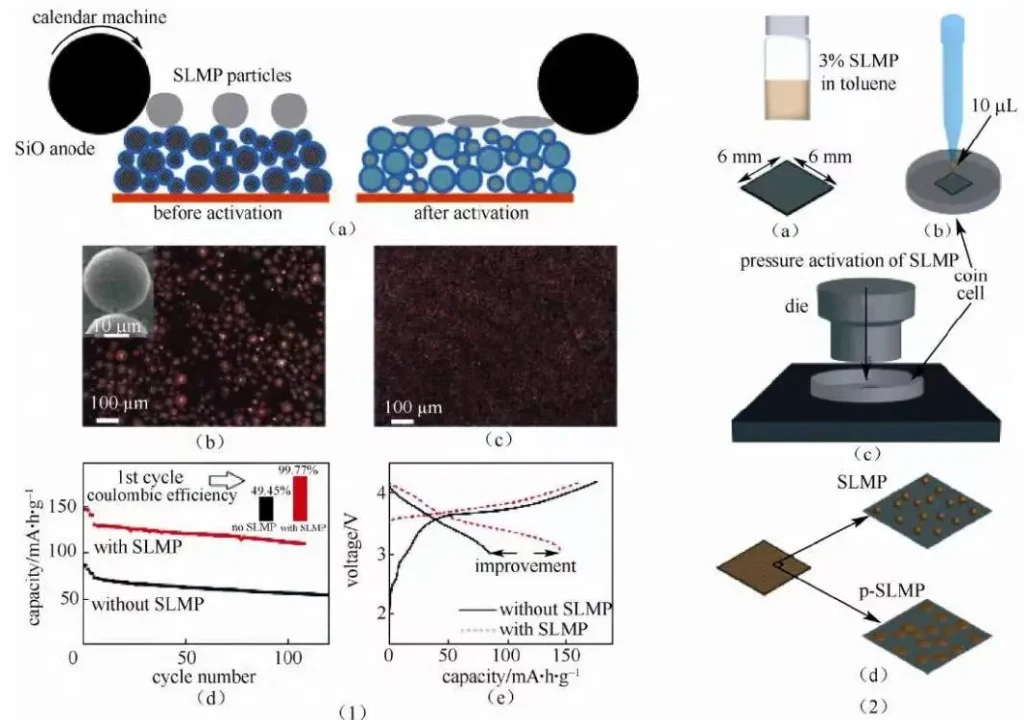
The use of lithium metal can not only directly interact with the anode material, but also indirectly form the pre – lithium – replenishment effect on the anode material during the initial cycle of the battery. Stabilized lithium metal powder (SLMP) is a kind of negative electrode prelithium additive produced and developed by FMC company in the United States. Because of the inert protective layer Li2CO3 on its surface, it has good stability in the air. Pan et al. disperse SLMP in hexane in advance to form a uniform dispersion solution, and then spray on the surface of the prepared polar sheet to form a uniform SLMP layer. After the solvent volatilizes and rolls, the protective layer of SLMP breaks, making the negative silicon carbon material directly contact with lithium.
After the initial cycle, ICE increased from 68.1% to 98.5%, and the capacity retention rate was 95% after 200 cycles, showing good cycle stability. Due to the lithium has good ductility, Cao and so on through the metal lithium in the copper foil surface pressure into a thin layer of metal lithium layer, then as a protective layer in the surface with a polymer coating to protect metal lithium Damage will not be in the air oxidation, anode materials are then coated on top of the preparation of the active material / 3 layer structure of polymer/lithium metal electrode materials. The polymer layer will slowly dissolve in the electrolyte, eventually allowing the lithium metal to contact with the graphite material to complete the pre-lithiation and lithium replacement. In this way, a high ICE value of 99.7% was achieved for the graphite negative and even more than 100% ICE in the silicon nanoparticle negative.
The results show that lithium metal has a good role of supplementing lithium, which can improve ICE, energy density and cycle stability of batteries. However, lithium has strong activity to water and oxygen in the air, and the protection process is made into a complex lithium supplement process, which increases the cost to the actual production. The uniformity of the lithium replenishment process of pre-lithium needs to be further improved, and the formation of lithium dendrites after excessive lithium replenishment caused by uneven lithium replenishment is also a technological problem that needs to be solved.
Due to its high activity, lithium metal is not conducive to electrode preparation. Similar to lithium metal, alloy complexes of lithium metal have low reduction potential and high capacity of lithium replenishment, which can be used as a substitute for lithium metal to realize lithium replenishment. However, the pure lithium alloy complex prepared by lithium metal, such as LixSi, has strong chemical activity, and will react quickly in the air exothermic reaction, so that direct use will still need complex protection engineering. Therefore, improving the chemical stability of lithium alloy is the key to make it become a rational prelibiation additive.
Zhao et al. prepared LixSi alloy by mechanical agitation of lithium metal and Si nanoparticles in accordance with a certain chemical and quantitative ratio, and then constructed Li2O oxide layer on the surface of LixSi with low oxygen content ratio in an inert atmosphere in the glove box. The core-shell LixSi-Li2O complex has certain stability in dry air, and LiXSi-Li2O can be used as a prelithium additive in polyvinylpyrrolidone electrode to increase ICE to more than 94%.
In order to further increase the stability of LixSi, Zhao et al. prepared LixSi/Li2O complex using low-cost SiO and SiO2. Due to the uniform distribution of Si and O atoms, the LixSi components are firmly embedded in the Li2O lattice generated from lithium, which makes them have good stability in the air with 40% humidity. Even if the structure of the surface LixSi collapses, the dense Li2O in the inner layer can still play a protective role. The low potential of the complex can achieve a good lithium replenishing effect on the anode material. As a pre-lithium additive, it can still provide a lithium replenishing capacity of 1 240 mAh/g after being exposed to the air for 6 h, and can still participate in the electrochemical cycle in the following cycle, showing a Coulomb efficiency of 99.87% in the 400 turn.
In addition to using silicon as the raw material for the preparation of lithium alloy compounds, Zhao et al. used the elements of the fourth main group (Z=Si, Ge, Sn) and the corresponding oxides to prepare alloy compounds Li22Z5 or Li22Z5-Li2O by one-step method. Li22Z5 or Li22Z5-Li2O alloy composites can play a good role of supplementing lithium for Sn base and graphite anode materials. According to chemical calculation, the binding energy of Ge and Li in LixGe is the highest compared with similar alloys, and it shows better stability in dry air. The dense Li2O lattice protection layer in LI22Z5-LI2O can greatly increase the stability of Li22Z5 in dry air, and the production process of direct mixing heating and stirring can reduce the cost of battery process improvement.
Compared with the high activity of lithium metal, the alloy compound LixZ of lithium has been greatly improved in stability, and some products can still maintain stability for 6 h in the air with 40% humidity. Moreover, the existence of Li2O lattice plays the role of skeleton support, so that the main active substance LixZ can still provide cycling capacity stably in the subsequent cycle process. However, the product can not be directly used in the water system sizing process of the negative mainstream, as a pre-libiation additive, because of its high activity. Therefore, it is of great practical significance to further improve the process of lithium alloy compound so that it can be directly used in water drainage slurry system.
Molecular clipping compounds of lithium metal dissolved in organic solvents have been studied extensively. However, in different reducing organic solvents, for silicon-based materials with low potential, the lack of reduction of organic solvents will lead to insufficient addition of active lithium in silicon-based materials. At the same time, the method has the characteristics of good stability, high safety and mild reaction, so choosing appropriate reagents for prelithium is one of the effective methods to eliminate irreversible capacity loss.
Yan et al. used biphenyl (Bp) and gold lithium to construct LiBp reagent in tetrahydrofuran solution. SiOx/C was heated, stirred and filtered to obtain LIBP-SiOX /C complex in this reagent. After heat treatment, LIBP-SiOx /C is transformed into LixSiOy and evenly dispersed in SiOx/C, which can effectively inhibit the irreversible consumption of lithium ions. The material has high capacity and cycle stability. As a negative material, the soft coated battery prepared by matching LinI0.8Co0.1Mn0.1O2 positive material has a high energy density of 301Wh /kg and a capacity retention rate of 93.3% after 100 cycles. Wang et al. prepared LiBp prelithiation solvent by dissolving lithium gold, biphenyl and tetrahydrofuran solutions, and its low reduction potential of 0.41 V can effectively reduce the active substances.
At the same time, the LiBp reagent has strong stability in a certain humidity air atmosphere, can increase the ICE of phosphorus and carbon electrode material to 94%, has certain industrial use value. Shen et al. by using naphthalene lithium as the prelithium reagent to prepare the prelithium nano Si electrode, reducing the irreversible capacity loss of about 1 500 mAh/g, so that the first week efficiency of Si electrode improved to 96.1%. The prelithium electrode and the corresponding Si/Li2S-PAN electrode were used to assemble the full battery with the first efficiency of 93.1%, and the energy density was as high as 710 Wh/kg. Naphthalene lithium reagents are safer and cheaper than conventional lithium reagents, and the depth of lithium can be controlled by controlling the temperature and time.
Compared with the single study on naphthalene lithium reagent, Jang et al. made Li+ reduction potential in organic reagents controllable by selecting a series of biphenyl organic reagents and introducing different functional groups at different benzene ring positions. Low reduction potential is beneficial for Li+ to participate in the SEI formation of silica-based anode materials, and it can also directly act on the lithiation process of silica-based anode materials in the process of prelithiation. By controlling the immersion time of the electrode material in the organic reagent of the system, the ICE of the material can be increased to nearly 100%.
Studies have shown that the organic lithium reagents constructed by molecular shear of organic reagents can form a good lithium supplement effect on negative electrode materials, even low potential silicon-based materials. However, the organic reagent itself is expensive and has certain toxicity, which has a certain cost of technological transformation for the existing battery production. Therefore, it still needs further technological improvement in the face of large-scale use.
The main material used in the anode of lithium ion battery is graphite. With the improvement of battery standards, the specific capacity and cycle life of the material need to be further improved. The pre-lithiation technology can further improve the overall energy density of the battery and reduce the loss of lithium ions during the first electrochemical cycle.
1) In metal lithium supplementing lithium, there are two ways to use metal lithium: direct contact and interjoint contact. The 3-layer electrode prepared by stabilized lithium metal powder and lithium foil calendering has been used commercially in bulk, but it has the disadvantages of uneven pre-lithiation and high cost. The lithium replenishment of metal lithium sheet involves the addition of control equipment of external circuit and the high time cost of lithium replenishment process, which is unfavorable to the demand of cost reduction in industrialization. Short circuit contact may face uneven lithiation phenomenon. Therefore, comprehensive advantages of various processes, the overall use of lithium metal in the lithium layer process still needs to be improved.
2) Lithium metal substitutes replace lithium with lithium alloy. Silicon lithium alloy compounds are added to the anode materials in the form of additives. However, due to their high activity, they are difficult to be stable in the air for a long time. However, for the direct use of water system slurry, the coating process still needs to be improved.
3) Lithium metal organic solvent, represented by lithium naphthalene reagent, has a low reduction potential and can play a good role in supplementing lithium to silicon-based materials with low potential. However, the actual lithium replenishment process involves equipment transformation and the increase of technological steps, which increases the difficulty of use to a certain extent. The expansion of application scope and the reduction of cost require further improvement of the process.
To find out more about our products and solutions, please fll out the form below and one of our experts will get back to you shortly
3000 TPD Gold Flotation Project in Shandong Province
2500TPD Lithium Ore Flotation in Sichuan
Fax: (+86) 021-60870195
Address: No.2555,Xiupu Road, Pudong, Shanghai
Copyright © 2023. Prominer (Shanghai) Mining Technology Co.,Ltd.
