

1.Hydrochloric acid and chloride salts
a.Hydrochloric acid
One of the most classic inorganic acids, it has good solubility for iron oxides and clay minerals. It is widely used because of its low price and obvious and intuitive effect. Whether it is pickling to remove the yellow skin in quartz plate, or the high-purity sand pickling, hydrochloric acid is favored.
The waste water treatment of hydrochloric acid is relatively simple. Neutralizing the solution with alkali to neutrality and reprecipitation can meet the national discharge standard. However, among the environmental protection cases of acid pollution in various places, the pollution of waste water containing hydrochloric acid is the most common.
Why?
Neutralization of hydrochloric acid wastewater requires consumption of alkali. Taking the most commonly used quicklime as an example, according to the chemical balance, the waste liquid produced by one ton of 31% industrial hydrochloric acid theoretically consumes about 0.25 tons of quicklime. In fact, because the quicklime is not fully dissolved, if 50% of the quicklime participates in the reaction, about 0.5 tons of quicklime is consumed for the waste liquid produced by one ton of industrial hydrochloric acid. The price of one ton of industrial hydrochloric acid is 100-400 yuan, the reference average price is 300 yuan; the price of one ton of quicklime is 400-1000 yuan, and the reference average price is 700 yuan. Then we can know that the cost of using one ton of hydrochloric acid is 300 yuan, the cost of lime for wastewater treatment is 350 yuan, and the cost of wastewater treatment has exceeded the cost of using hydrochloric acid. Some non-standard enterprises, on the one hand, do not have wastewater treatment facilities, and on the other hand, they are unwilling to bear higher costs, resulting in frequent occurrence of waste acid direct discharge pollution incidents.
After all, Marx said: “For 100% profit, capital dares to trample all human laws”.
b.Chloride salts
Common chloride salts, such as sodium chloride, potassium chloride, lithium chloride, calcium chloride, magnesium chloride, can be used for doping and purification of quartz sand, and can also be used for chlorination roasting and whitening in non-metallic minerals such as kaolin.
Some literatures mention chlorination and purification of quartz sand with ammonium chloride, hydrogen chloride, chlorine or carbon tetrachloride.
2.Sulfuric acid and sulfate
Binary inorganic acid with strong oxidizing property and high boiling point. The boiling point of concentrated sulfuric acid is 338 °C, and it is non-volatile under normal conditions, so it is not as widely used as hydrochloric acid in applications requiring acid mist treatment. The advantage of high boiling point is that the minerals can be processed before heating to the boiling point (such as around 300°C) without using a high-pressure vessel. Such extreme conditions can decompose some minerals that cannot be dissolved by hydrochloric acid. Of course, this situation has high requirements on materials and safety protection, which is rarely seen in actual production, but more in the laboratory.
Some literatures mention the use of salts of sulfuric acid and calcination of quartz sand to reduce the titanium content of quartz sand. Treatment with ammonium salts of sulfuric acid reduces the iron content of quartz sand.
The acid wastewater treatment of sulfuric acid and sulfate is the same as hydrochloric acid wastewater, which can be neutralized with alkali.
3.Hydrofluoric acid and fluoride salts
Monobasic weak acid hydrofluoric acid, with its super complex ability, has become a big killer for quartz sand purification. Under certain conditions, hydrofluoric acid reacts with most impurity minerals, including quartz sand. Therefore, when the concentration of hydrofluoric acid is too high, it is necessary to pay attention to the loss of quartz sand. Mixed acids of hydrofluoric acid and hydrochloric acid, sulfuric acid or nitric acid are commonly used mixed acid systems. In the field of oilfield, graphite, silicon carbide and other non-metallic minerals adopt mixed acid system containing hydrofluoric acid.
The role of fluoride salts in acid-containing systems is similar to that of hydrofluoric acid. Fluoride salts are also used as dopants.
The pearls produced by human industrial civilization are inseparable from the existence of hydrofluoric acid. In the semiconductor industry, hydrofluoric acid is mainly used to clean the wafer surface, or in the process of cleaning and etching during chip processing. In the solar industry, hydrofluoric acid is used in processes such as chip surface cleaning and etching. In the panel industry, hydrofluoric acid is used for cleaning glass substrates and etching silicon nitride and silicon dioxide. However, in the high-purity quartz sand industry, some people try to find a “fluorine-free” or even “acid-free” solution. Is this scientific?
In addition to alkali neutralization, the most important point of hydrofluoric acid wastewater treatment is to reduce the fluoride ion concentration to the range permitted by the national standard. The overall treatment process is not complicated, and regular enterprises are capable of handling hydrofluoric acid wastewater. However, some small and scattered enterprises do not have professional wastewater treatment facilities and are unwilling to increase the treatment cost, and the direct discharge of wastewater causes environmental pollution. If the wastewater is directly discharged without treatment, it is easy to cause the fluorine content in the water area to exceed the standard, which is also the main reason for the discoloration of fluorine in some places.
4.Phosphate and Phosphate
Ternary medium strong acid, boiling point 261℃ (decomposition). Concentrated hot phosphoric acid can decompose most minerals, such as chromite, rutile, ilmenite, etc., and can also react with silica to form heteropolyacids. Phosphoric acid is the only acid other than hydrofluoric acid that can react with quartz.
The normal salt and acid salt of phosphoric acid can also be seen in the corrosion experiments of quartz materials.
The wastewater treatment of phosphoric acid and phosphates needs to be neutralized with alkali first, and then the concentration of phosphates needs to be reduced to the range permitted by the national standard.
5. Nitric acid and nitrates
Nitric acid is an inorganic strong acid with strong oxidizing properties. For some reducing mineral impurities, the effect of conventional acid systems is limited, some reactions do not occur, and some reactions that are chemically thermodynamically feasible are kinetically hindered. At this time, if a strong oxidant is involved, the reaction can be carried out and the reaction speed can be greatly accelerated. And because nitrates generally have higher solubility, the addition of nitric acid prevents the precipitation of reaction products. The mixed use of nitric acid and other acid systems is suitable for the treatment of quartz sand containing reducing minerals.
The role of nitrate in acid-containing systems is similar to that of nitric acid. Nitrate is also used as a dopant.
In the wastewater treatment of nitric acid and nitrate, in addition to neutralization with alkali, measures should also be taken to reduce the ammonia nitrogen content in the wastewater.
1.Oxalic acid
The binary organic is strong, and its acidity is medium strong acid, which is a strong acid in organic acids. Oxalate has a strong coordination effect and is an effective metal chelator. In the iron removal experiment of quartz sand, the use of oxalic acid alone, or the combination of oxalic acid and ultrasonic waves, or the combination of oxalic acid and other acid systems, can achieve a better effect of iron removal and whitening. There are also a lot of reports mentioning that oxalic acid is used in the purification and bleaching of non-metallic minerals such as kaolin. Moreover, the amount of oxalic acid does not need to be as large as that of traditional inorganic acids such as hydrochloric acid, and only needs not more than 5% to achieve the maximum pickling effect. Oxalate will combine with calcium and magnesium ions to form precipitates with low solubility, so oxalic acid has certain limitations when dealing with minerals with high alkaline earth metal content.
In oxalic acid wastewater, in addition to the influence of acid, the presence of oxalate as an organic matter will also greatly increase the chemical oxygen demand of the water body. Therefore, lime treatment is the preferred solution. In addition to neutralizing acidity, oxalic acid can also be precipitated to greatly reduce the residual oxalate content.

2.Citric acid and sodium citrate
Citric acid is a tricarboxylic acid compound and is an important organic acid. Citric acid is weaker than oxalic acid, but is a strong acid among organic acids. Citric acid and its salts have strong chelating ability in the acidic range, and can chelate most of the trivalent and trivalent metal ions. The suitable use range is pH=4~8. The chelate formed by citric acid and iron ion has low solubility and will form a precipitate in water. In order to increase its solubility, an appropriate amount of ammonium salt is added to form a compound with higher solubility.
The biggest difficulty in wastewater treatment of citric acid and other organic compounds is the reduction of chemical oxygen demand. A large amount of organic matter enters the wastewater, which will cause the chemical oxygen demand to soar. The reduction of chemical oxygen demand requires professional equipment and sites, such as chemical oxidation pools and biological oxidation pools, whose capital investment and processing difficulty are far greater than those of acid-base neutralization facilities.
3.EDTA (ethylenediaminetetraacetic acid) and its sodium salt
EDTA and its sodium salt are important complexing agents, which have a wide range of coordination properties and can form stable chelates with almost all metal ions. It is used in a neutral and weak alkaline environment, and has poor corrosion ability. It is suitable for the removal of clay minerals and thin-film iron oxide impurities.
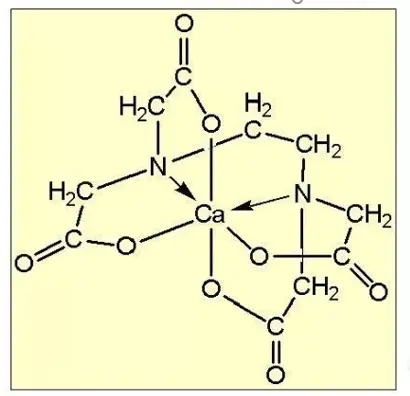
4.Other complexing agents
Such as acetic acid, salicylic acid, organic polyphosphonic acid, etc., the acidity is relatively weak, but the complexing ability is outstanding, and it can be used as a complexing agent.
Whether there is a better solution for the chemical treatment of quartz sand is still unknown. And each substance has corresponding advantages and disadvantages, usually several substances are mixed to achieve the best effect. The combined use effect of various substances and whether the drug regimen matches the purpose of treatment are all factors that we need to consider when dealing with quartz sand. I hope everyone can adapt to local conditions and use the most suitable drug regimen.
To find out more about our products and solutions, please fll out the form below and one of our experts will get back to you shortly
3000 TPD Gold Flotation Project in Shandong Province
2500TPD Lithium Ore Flotation in Sichuan
Fax: (+86) 021-60870195
Address: No.2555,Xiupu Road, Pudong, Shanghai
Copyright © 2023. Prominer (Shanghai) Mining Technology Co.,Ltd.
