

The general beneficiation process of domestic quartz sand purification has developed from “grinding, magnetic separation, washing” in the early stage to “sorting → coarse crushing → calcination → water quenching → grinding → screening → magnetic separation → flotation → acid leaching →washing →drying”, combined with microwave, ultrasonic and other means for pretreatment or auxiliary purification, the purification effect has been greatly improved.
In view of low-iron requirements of photovoltaic glass, the iron removal methods from quartz sand are mainly concerned.
Generally, iron exists in the following six common forms:
① Occurs in the form of fine particles of clay or kaolinized feldspar
② Attaches to the surface of quartz particles in the form of iron oxide film
③ Iron minerals such as hematite, magnetite, specularite, tinite, etc. or iron-bearing minerals Mica, amphibole, garnet, etc.
④In a disseminated or lens state inside of quartz particles.
⑤In the state of solid solution inside the quartz crystal.
⑥Mixed in during the crushing and grinding process.
To effectively separate iron-bearing minerals from quartz, it is necessary to first prove the occurrence state of iron impurities in quartz ore and select a reasonable beneficiation method to remove iron impurities.
(1) Magnetic separation process
The magnetic separation process can maximally remove weak magnetic impurity minerals such as hematite, limonite and biotite including conjoined particles. According to the magnetic strength, magnetic separation can be divided into high intensity magnetic separation and low intensity magnetic separation, among which high intensity magnetic separation usually adopts wet high intensity magnetic separator or high gradient magnetic separator.
Generally speaking, for the quartz sand containing impurities mainly weak magnetic impurity minerals such as limonite, hematite, biotite, etc., it can be selected by using a wet magnetic machine above 8.0×105A/m; For strong magnetic minerals dominated by iron ore, it is better to use a weak magnetic machine or a medium magnetic machine for separation.
With the application of high-gradient magnetic field magnetic separator, the purification of magnetic separation is obviously improved compared with the past. For example, under the magnetic field strength of 2.2T, the iron removal by electromagnetic induction roller type strong magnetic separator can reduce the content of Fe2O3 from 0.002% to 0.0002%.
(2) Flotation process
Flotation is the process of separating mineral particles by their different physical and chemical properties on their surfaces, and the main function is to remove the related minerals mica and feldspar from quartz sand. For the flotation separation of iron-bearing minerals and quartz, finding out the occurrence form of iron impurities and the distribution form in each particle size is the key to choosing an appropriate sorting process for iron removal. Most of the iron-containing minerals have a zero electric point above 5, and are positively charged in an acidic environment. In theory, anionic collectors are suitable.
Fatty acids (soaps), hydrocarbyl sulfonates or sulfates can be used as anionic collectors for flotation of iron oxide ores. For pyrite, the classic floating sulfur agent is isobutyl xanthate plus butylamine black (4:1), the dosage is about 200ppmw, and pyrite can be floated from quartz in the pickling environment.
In flotation of ilmenite, sodium oleate (0.21mol/L) is generally used as a flotation agent, and the pH is adjusted to 4~10. A chemical reaction occurs between oleate ions and iron particles on the surface of ilmenite to produce iron oleate. Oleate ion keeps ilmenite well floatable. Hydrocarbon-based phosphonic acid collectors developed in recent years have good selectivity and collection performance for ilmenite.
(3) Acid leaching process
The main purpose of the acid leaching process is to remove soluble iron minerals in the acid solution. The factors affecting the purification effect of acid leaching include quartz sand particle size, temperature, leaching time, acid type, acid concentration, solid-liquid ratio, etc. The leaching rate can be improved by the temperature, concentration and decreasing the radius of quartz particles.
The purification effect from single type of acid is limited, and the mixed acid has a synergistic effect, which can greatly improve the removal rate of impurity elements such as Fe and K. Common inorganic acids are HF, H2SO4, HCl, HNO3, H3PO4, HClO4, H2C2O4, we could adopt two or more mixed in a certain proportion.
Oxalic acid is an organic acid commonly used in acid leaching. It can form a relatively stable complex with the dissolved metal ions, and impurities can be easily washed out. Some people used ultrasonic-assisted oxalic acid purification, and found that compared with conventional stirring and tank ultrasonics, probe ultrasonics had the highest removal rate of Fe, the dosage of oxalic acid was less than 4g/L, and the iron removal rate reached 75.4%.
The coexistence of dilute acid and hydrofluoric acid can effectively remove Fe, Al, Mg and other metal impurities, but the amount of hydrofluoric acid should be controlled, because hydrofluoric acid can corrode quartz particles. The use of different kinds of acids also affects the quality of purification. Among them, the processing effect of HCl and HF mixed acid is the best. Some people use HCl and HF mixed leaching agent to purify the magnetically separated quartz sand. Through chemical leaching, the total amount of impurity elements is 40.71μg/g, and the purity of SiO2 is as high as 99.993wt%.
Some researchers used kaolin tailings to prepare low-iron quartz sand for photovoltaic glass. The main mineral composition of kaolin tailings is quartz, with a small amount of impurity minerals such as kaolinite, mica and feldspar. After the kaolin tailings are processed by the beneficiation process of “grinding – hydraulic classification – magnetic separation – flotation”, the 0.6~0.125mm particle size content is greater than 95%, SiO2 is 99.62%, Al2O3 is 0.065%, Fe2O3 is 92×10-6. Processed quartz sand meets the quality requirements of low iron quartz sand for photovoltaic glass.
Shao Weihua from Chinese Academy of Geological Sciences, published an invention patent: a method for preparing high-purity quartz sand from kaolin tailings.
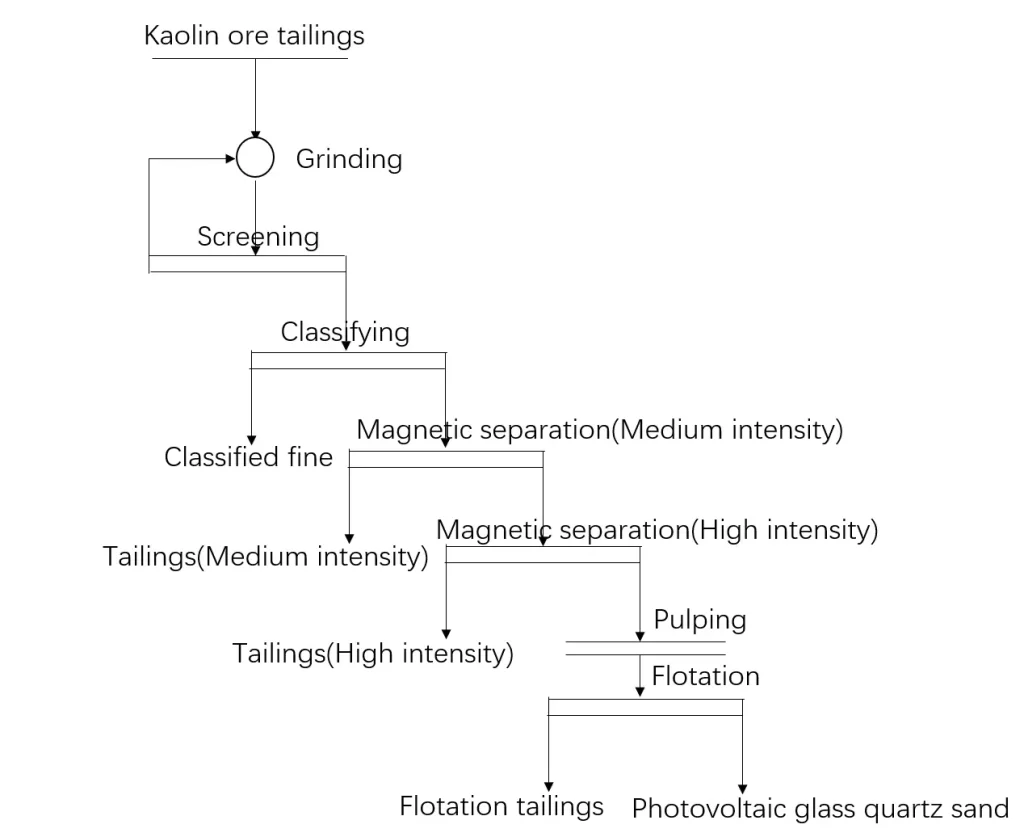
The method steps:
a.kaolin tailings are used as raw ore, and after stirring and scrubbing, the +0.6mm material is obtained;
b.the +0.6mm material is graded after grinding, and the 0.4mm-0.1mm ore material is subjected to magnetic separation operation, obtain magnetic and non-magnetic substances, non-magnetic substances enter the gravity separation operation, obtain gravity separation light minerals and gravity separation heavy minerals, gravity separation light minerals enter the regrinding operation for screening, and obtain +0.1mm minerals;
c.+0.1mm minerals enter the flotation operation to obtain a flotation concentrate. The flotation concentrate removes the upper layer of water and then undergoes ultrasonic pickling, and then sieving to obtain +0.1mm coarse material as high-purity quartz sand. The method of the invention can not only obtain high-quality quartz concentrate products, but also shorten the processing time, simplify technological process and reduce energy consumption.
Kaolin tailings contain a large amount of quartz resources, which can meet the requirements of photovoltaic ultra-white glass raw materials through beneficiation, which also provides new ideas for recycling utilization of kaolin tailings resources.
To find out more about our products and solutions, please fll out the form below and one of our experts will get back to you shortly
3000 TPD Gold Flotation Project in Shandong Province
2500TPD Lithium Ore Flotation in Sichuan
Fax: (+86) 021-60870195
Address: No.2555,Xiupu Road, Pudong, Shanghai
Copyright © 2023. Prominer (Shanghai) Mining Technology Co.,Ltd.
